Researchers investigate how lasers can lead to cleaner crystallization process
Microscopic experiments could make massive industrial processes more sustainable: researchers investigate laser-induced crystallization using microfluidics.
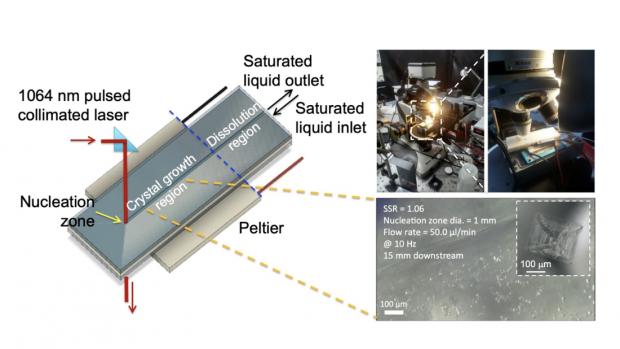
This image, taken in the laboratories of Bruce Garetz and Ryan Hartman, shows how the researchers used a purpose-designed microfluidic “lab on a chip” setup paired to a 1064 nanometer pulsed laser to examine laser-induced nucleation of organic molecules, leading to crystallization.
When Bruce Garetz, professor of chemical and biomolecular engineering, examined the test tubes that his student had exposed to laser light in his lab, he noticed something odd: The tubes that once contained liquid solution now contained what appeared to be crystals, implying that nucleation and crystal growth, two steps of the process of crystallization, had occurred.
Why had this happened? After scouring scientific literature for explanations, he found no answers. Now Garetz and colleague Ryan Hartman, associate professor of chemical and biomolecular engineering, will have a chance to pull the curtain back on this process. On June 16, 2021, the pair received a $453,103 grant from the National Science Foundation (NSF) to design and study microfluidic non-photochemical, laser-induced nucleation (NPLIN) of preselected organic molecules.
Crystallization is more than just visually impressive — it is a process that forms the basis for thousands of industrial chemical mechanisms essential to the manufacture of pharmaceuticals, dyes, food additives, even explosives. Understanding laser-light induced crystallization could lead to more sustainable industrial processes.
Present industrial methods to induce crystallization are energy-intensive and consume enormous quantities of chemical solvents. Understanding and controlling crystallization through exposure to laser light could reduce reliance on these solvents, leading to a more efficient, and ultimately “greener” crystallization process for some applications.
To understand the phenomenon, Garetz embarked on a detailed, quantitative analysis of the nucleation process, in collaboration with an expert in microfluidics. Hartman’s research, for which he was awarded an NSF CAREER award for young researchers in 2015, involves finding new ways of investigating phenomena — such as those involving phase transitions — through the design and use of microfluidic devices that allow for the examination of nucleation using extremely small volumes of reagents. The researchers, by isolating each crystal in the solution and tracing its charges, can examine in situ what is happening within a complex chemical process.
One benefit of microfluidics is that the practice allows researchers and manufacturers to investigate and optimize processes without excessive waste of material and energy. As Hartman discussed in an article on microfluidic experimentation for the design of polymers, chemical companies typically use large quantities of reagents to examine processes.




